Place 내용
-
Hanmi Bio Plant
Bio Plant Complex
Established in 2007, Hanmi Bio Plant has been fully devoted to manufacturing biological medicine with highest quality.
We are experts in manufacturing and development of recombinant protein products based on microbial fermentation and biochemical conjugation reaction processes. Our current pipeline includes more than 10 biologics and medical device products in various clinical phases and commercialization.
In 2008, Hanmi Bio Plant received KGMP certification as well as Hyaluronate product approval. In 2011, its facility and Hyaluronate-based product have gained ISO 13485 certification from SGS. In 2018, our product obtained US FDA PMA approval as a Class 3 medical device product.
-
Hanmi Bio Plant Complex – Best-Fit CMO/CDMO Facility
 Located in Pyeongtaek, South Korea (50 miles south of Seoul)Clinical to Commercial Scale Manufacturing and Development
Located in Pyeongtaek, South Korea (50 miles south of Seoul)Clinical to Commercial Scale Manufacturing and Development- 2007 Established and KGMP accredited
- 2018 US FDA PMA approval of Synojoynt™ (Hyaluronate Sodium Injection, Medical Device for Osteoarthritis Joint Pain)
- 2020 US FDA BLA approval (Rolontis®) pending FEI No. 3009350213
We are capable of manufacturing recombinant proteins, plasmid DNA, mRNA, including template DNA production, in-vitro transcription to purification in flexible scales
- E.coli fermentors: 300 L ~ 10,000 L x2 (largest in Korea)
- Biochemical reactors: flexible up to 500 L or 7,500 L
- Purification: flexible up to 3 kg / batch by Centrifugation, Chromatography, UF/DF TFF
Commercial-ready, state-of-the-art, CGMP manufacturing facilities
Experience in HA inspection with US FDA-standard Quality System
Expanded manufacturing capacity enabling dedicated production facility
On-site R&D resources available (Process Dev, Analytical Dev and CMC RA)
Reliable/quality raw materials sourcing by Hanmi Fine Chemical Co.
-
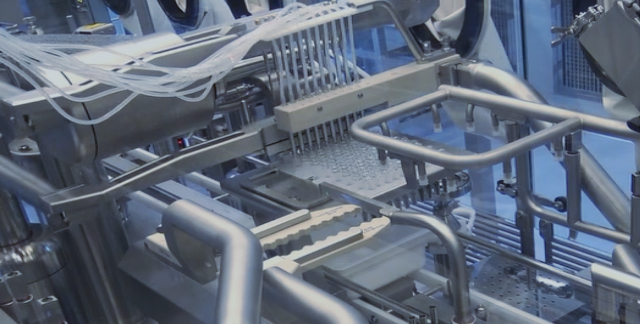
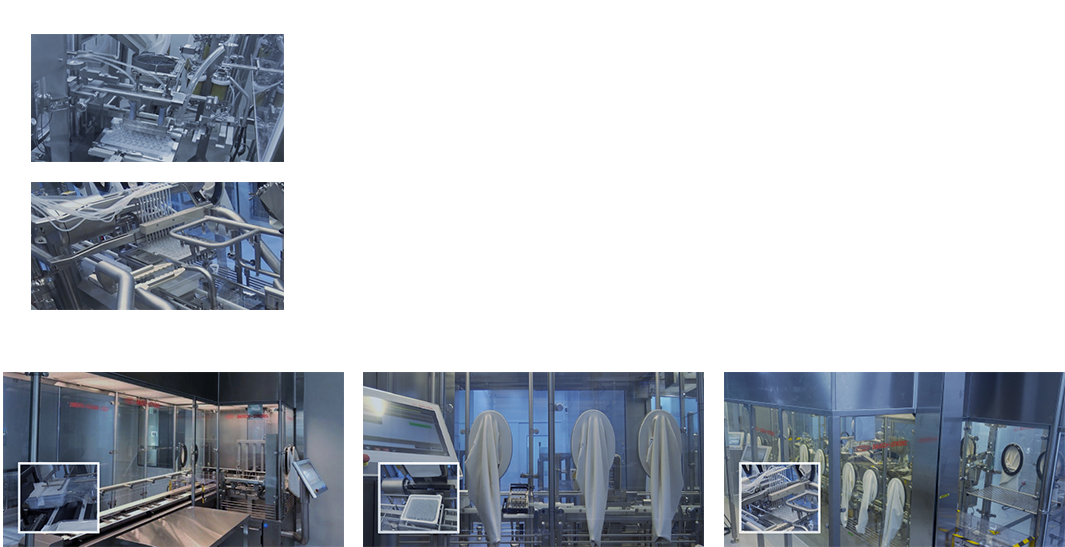
Production Building 1 & 2
Our Advantages
Rapid Lab-to-Pilot DevelopmentCommercial-ready, CGMP FacilitiesUS FDA-standard Quality Management SystemInnovative Process Development CapabilityBio CMC Regulatory ExpertiseCo-development Experiences with Global Partners
-
Accelerated Pathway from Lab to Plant(APLP SolutionsTM) Workflow
-
Upstream Process (Fermentation & Recovery)
Our fermentation experts provide exceptional development support for cell culture processes that meet future commercial requirements through systematic optimization of parameters


- 12 parallel 1L glass fermentors
- 3 parallel Sartorius 30L stainless steel fermentors
- Controlled temperature cabinets for flask cultures
- Freezers for WCB / harvest / intermediate
-
Process Development
Downstream Process (Purification)Biochemical Reactions (PEGylation and Conjugation)
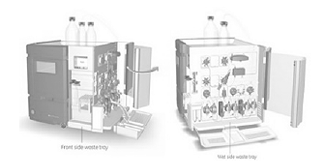
- Parallel reactors of various scale
- Lab and pilot scale chromatography controllers (AKTA Avant, AKTA Pure, AKTA Pilot)
- Lab scale chromatography columns: 6.6 – 50 mm (XK Column, LRC Column)
- Pilot scale chromatography columns: 50, 70, 100, 140, 200, 300, 450, (BPG Column, AxiChrom, Quickscale, Fineline Column )
- Lab and pilot scale UF/DF systems (TFF System, MF system)
- Cell Disruptor & POD System
We use scientifically sound techniques including design-of-experiments approach for process optimization/characterization to achieve robust and economic processes. We map product quality and impurity reductions throughout development life cycle to generate superior quality products. Every step we take towards an efficient, scalable manufacturing process will deliver consistent product quality.
Reliable and robust analytical methods are essential for successful drug development and commercialization.
Our analytical capabilities help understand and characterize your molecule in a phase-appropriate manner.
Services Provided:
- Defining product quality attributes
- Analytical method development, Qualification, and Validation
- Product characterization services (Primary and high order structure, Post-translational modification, Glycan
- Disulfide bridge determination, product related impurities, in-vitro biological activity)
- GMP testing services (Batch release, stability, raw materials, and microbial tests)

-
Analytical capability to support research and bio analytical characterization
-
Integrated efforts to develop analytical methods for process characterization and various assays
-
Method Development for the in-process testing to ensure product quality during manufacturing
-
Method development to improve reliability and robustness for commercial product testing
-
Preparation of CMC regulatory documentation package for submission and GMP inspection support

Physico-Chemical Analyses
In-Process Test
DS/DP Release Test (Identity, purity & impurities, potency, and process/product residuals)
Stability Test (Long-term, accelerated, stress, photo-stability)
Analytical Method Validation
Raw Materials Analyses
Compendial Test according to pharmacopoeia (USP/ EP/ BP/ KP)
Analytical method verification for compendial method and validation for non-compendial method
Cell-Based Assay
In-vitro Bioassay: Cell Proliferation & Product-specific Cell-Based Assay
Assay Readouts: Absorbance, Fluorescence, Luminescence, Time-resolved fluorescence, Luminescence
Statistical Evaluation: Parallel Line Analysis, EC50 Determination, Equivalence Test
Microbial Test Method Development & Support
Microbial Enumeration Test (TAMC, TYMC, Specific Microorganisms)
Sterility Test (Isolator)
Endotoxin Test (Kinetic, Gel-Clot)
Disinfectant Efficacy Test Development
Microbial Identification Test (16s rRNA, MALDI-TOF system)
Electronic Data Management System maintained according to US FDA 21 CFR Part 11
- LIMS
-
Laboratory
Information
Management
System -
Test Management
- Test requested through the SAP interface
- Test request and reception
- Test result input and Review/Approval
- Print report -
Laboratory Execution System
- Automating Laboratory Equipment Data
- QR code systemStock / Maintenance / Trend
- Stock Management
- Equipment Management
- Trend
- UMS
-
Utility
Monitoring
System -
Utility (EM & Water) Monitoring Data Management
- Sample Schedule & Execution
- Response to Environmental Contamination
→ Reduces Contamination Risk
- Analyze Utility / Environmental Trend
→ Removes Potential Risk
- LEMS
-
Laboratory
Equipment
Management
System -
Monitoring Chamber Condition
- Paperless digital recorder displays real-time measured data
- Alert management
- Automatic data storage backup
- Back-up System
-
Back-up of All Data Generated
- Primary and Secondary Back-up - Automatic Daily Back-up
- Empower – Back-up from Chromatographic Systems
- NuGenesis - Back-up from Standalone Instruments
-

Collaboration Opportunities:
- Commercial/clinical contract manufacturing for recombinant protein, protein/peptide conjugates, pDNA, mRNA
- Co-development for process improvement and CoGs reduction
- Strategic sourcing of key raw materials
Please send inquires to the email address for business development with HanmiScience
E-mail : bd@hanmiscience.co.kr
-
Please send inquires for business
development with Hanmiscience
E-mail : bd@hanmiscience.co.kr